Articles
- Details
- Category: Articles
In this assignment, I observe and record the growth of peas and radishes over the course of seventeen days.
NOTE: Seeds were checked everyday for drying, mold and bacterial growth, and general health. For the purposes of time, no entries were made on certain days. The napkins/soil were also checked every day to make sure the plants had sufficient moisture. No hard and fast rule was used to determine if paper or soil was too dry or needed moisture. The medium was felt and judged accordingly. At no time was a pool of water present with the plants in the container. Neither was the container devoid of water at any time.
February 21, 2018 - 7:00 PM – (Time spent: 90 minutes setting up seed container and seeds, finding suitable place to keep them, taking pictures and making observations)
- Details
- Category: Articles
This is a Chemistry lab designed to illustrate the process of electrolysis, defined as chemical decomposition produced by passing an electric current through a liquid or solution containing ions.
Theory:
The first experiment involved the creation of a galvanic cell out of an aluminum can and scraps of copper wire, which was used as an energy source to power various small electronic devices. A galvanic cell is an electrochemical cell that is produced by a spontaneous redox reaction. In this case, the spontaneous reaction is between aluminum, the reducing agent, and copper, the oxidizing agent.
- Details
- Category: Articles
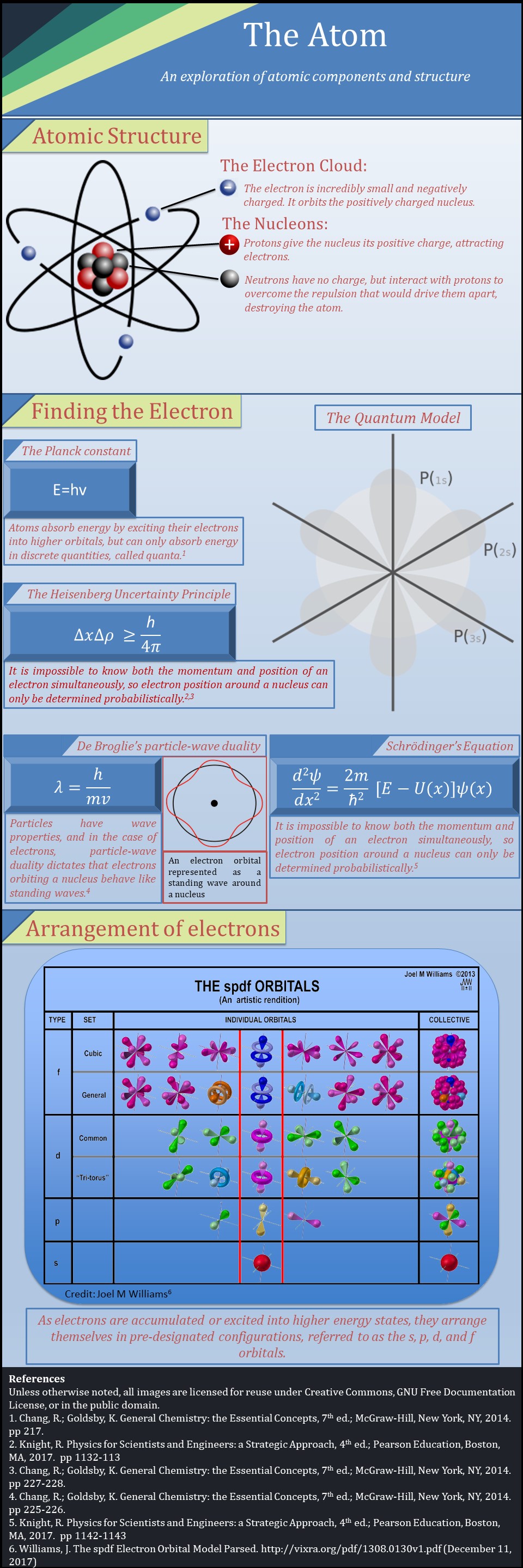
This infographic describes the basic properties of atomic structure, as well as the laws and equations that describe the subatomic particles.
- Details
- Category: Electrolysis Lab
The purpose of experiment 1 was to create a functioning battery using an aluminum can and copper wire. This was done successfully, and a voltage of 0.620V was measured using a voltmeter. This voltage was much less than the expected value of 2.00V, which was determined through calculation of standard cell potential equation: E°cell = E°cathode – E°anode. The total error between the measured and expected voltage is 69%.
The second experiment was meant to measure the volumes of gas generated at the anode and cathode of a system by the electrolysis of water, and to determine what molecules are present at each point. To this end, a theoretical hydrogen to oxygen ratio of 2:1 was calculated based on the balanced equation. However, the measured gas volume ratio was 2.15:1. This ratio was only 7.5% different from the expected value. Given the similarities between the two ratios, it can be determined that hydrogen gas had been generated at the anode and that oxygen gas had been generated at the cathode. This is corroborated by the color of the aqueous solution at each cathode. If hydrogen gas is generated at the cathode, it is to be expected that the aqueous solution would have a lower proton concentration, and thus, would have the bluer color associated with a higher pH. Similarly, if oxygen gas is generated at the anode, it would be expected that the aqueous solution be left with a higher proton concentration, and thus have the redder color associated with a low pH.
- Details
- Category: Electrolysis Lab
There appears to be a great deal of error in the first experiment, evidenced by the 69% difference between the expected and experimental E°cell values. After the construction of the battery, the copper wire touched the aluminum can, shorting out the battery for around a minute. This may be a contributing factor in the unexpectedly low battery voltage. Additionally, the theoretical E°cell value presupposes a temperature of 298 K. The temperature was not measured during the experiment, but it is unlikely that the temperature was at the theoretical level. This may have led to a lower reduction potential. Another possible source of error could have been in the accuracy of the voltmeter used. It was not calibrated against a known source.
- Details
- Category: Electrolysis Lab
Part 1:
| Initial Observations of Al can/copper wire battery: Dirty copper wire, clean and shiny on top, copper color, dirty part is whitish green. Initial voltage: 0.662 V |
|
Observations of Al can/copper wire battery after 20 minutes: Looks the same, Cu wire, turned pink under solution. 0.620 V. Bubbly pinkish brown stuff formed on submerged part of the can. Submerged can is very thin and fragile. Wire is significantly cleaner. |
